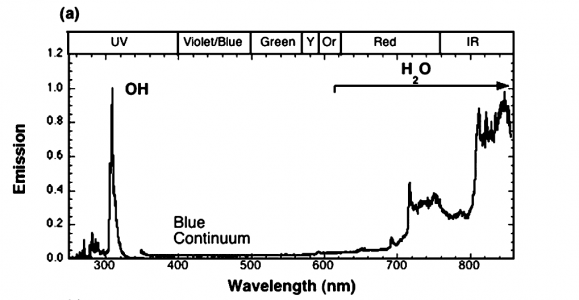
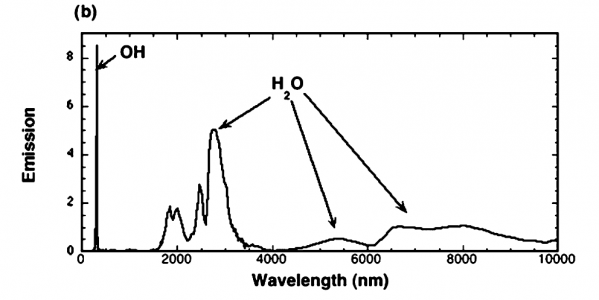
The article, written at the Combustion Research Facility at Sandia National Labs, states the figure is combustion. The paper is 8 pages, and contains some interesting pictures. It is worth a quick read, in my opinion. The article states the test conditions.
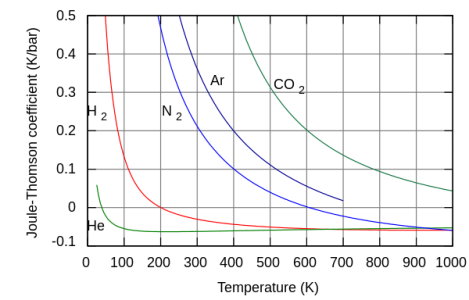
The Joule Thompson effect is entirely different. Leaking compressed room temperature hydrogen heats, depending on the pressure ratio. Very low relative pressures, like found in a laboratory burner, will only slightly heat. Like in a bunsen burner. A 0.5 psi leak is entirely different hazard than say a 1000 psi leak. The low pressure leak will result in flammability or explosion hazard issues as a result of the leak. High pressure leaks tend to self ignite, perhaps after filling the area with hydrogen. The flame is practically invisible in daylight. The open flame could cause other issues in an industrial environment.
Can't profess to be an expert, but my father did work with hydrogen, at the Radiation Laboratory at MIT during and after WWII. I learned a bit about hydrogen safety from him. As a technician in the lab, he fabricated some of the early magnetrons and similar microwave devices. H also worked on the linear accelerator there. He did all sorts of fabrication using all sorts of materials, trying to aid the war effort. Built and used his own hydrogen brazing furnaces at MIT. Later on, after attaining his degrees, he continued to work at MIT.