-
Welcome back Guest! Did you know you can mentor other members here at H-M? If not, please check out our Relaunch of Hobby Machinist Mentoring Program!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- Joined
- Mar 3, 2014
- Messages
- 634
Have used dry ice in a alcohol bath for shrinking parts that can be submerged. Alcohol is cheaper and safer than acetone.
It will create a fog but is far more efficient than just contact with the dry ice.
Look at the expansion or shrink rates [ machinist handbook ] for the materials and the interference fit. Do the math to determine how much thermal difference is needed.
Plenty of people would help with the formulas but need the material types and the fit dimensions. Alloys matter.
It will create a fog but is far more efficient than just contact with the dry ice.
Look at the expansion or shrink rates [ machinist handbook ] for the materials and the interference fit. Do the math to determine how much thermal difference is needed.
Plenty of people would help with the formulas but need the material types and the fit dimensions. Alloys matter.
B
BRIAN
Forum Guest
Register Today
Thanks for all the input In days gone bye when I worked in a R&D lab we used to freeze things in Dry ice and Tricoetheline
Looking back on it I wonder I am still here today
However living on top of a mountain in Sicily in not the best place to find anything mildly exotic,
Even the plumbers spray will probably have to come from the UK.
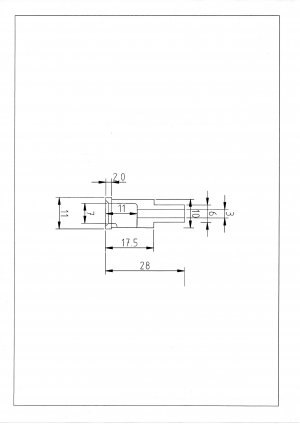
Attached is a drawing of the part I will leave more material on the inside dim's and final size in the head.
The cage material is Colphos similar to P102 but better machinability . the head is 6082
I have not given thought to tolerances yet so your opinions will help.
The cage is under no mechanical stress, the fit is for location only.
I have 10 to make and fit. The 17.5 x 10 dimension is the one involved
Brian.
Looking back on it I wonder I am still here today
However living on top of a mountain in Sicily in not the best place to find anything mildly exotic,
Even the plumbers spray will probably have to come from the UK.
Attached is a drawing of the part I will leave more material on the inside dim's and final size in the head.
The cage material is Colphos similar to P102 but better machinability . the head is 6082
I have not given thought to tolerances yet so your opinions will help.
The cage is under no mechanical stress, the fit is for location only.
I have 10 to make and fit. The 17.5 x 10 dimension is the one involved
Brian.

- Joined
- Dec 18, 2013
- Messages
- 2,012
I owned a German made Mercury Capri years ago. It had a 60 degree V8 with a timing gear made of nylon teeth. These would get old and brittle and go BOOM into shrapnel. The replacement timing gear which was about 8 inches in diameter was all aluminum. It was an interference fit on the camshaft. The directions were to boil it in a pot of water then slip it on. I was surprised how loose it was after boiling, it seemed pretty sloppy but after it cooled it had a death grip on the shaft. So for aluminum I would be more comfortable boiling it in water vs a torch or some form of direct heat.
Freezing parts then rapidly warming them seems unwise.
Freezing parts then rapidly warming them seems unwise.
- Joined
- Oct 21, 2014
- Messages
- 2,156
I've use the oven many times to heat aluminum for projects. The nice thing about it is you can put it in for a fairly long period to heat all the way through where boiling it you have to suspend it in the water (if you leave it on the bottom of the pan, it gets hotter on the bottom than the top. But to each may be different. ALso I can do bigger parts in a standard oven than I can in a pot.
- Joined
- Dec 18, 2013
- Messages
- 2,012
I've use the oven many times to heat aluminum for projects. The nice thing about it is you can put it in for a fairly long period to heat all the way through where boiling it you have to suspend it in the water (if you leave it on the bottom of the pan, it gets hotter on the bottom than the top. But to each may be different. ALso I can do bigger parts in a standard oven than I can in a pot.
You just use the wife's stainless double boiler to suspend parts
- Joined
- Feb 17, 2013
- Messages
- 4,407
I've never tried boiling, but it sound workable. The disadvantages are in finding a vessel large enough, plus the fact that you're limited to just one temperature. On the advantage side, water has hugely more heat capacity than any gaseous medium, so it will transfer heat into the part very rapidly (and evenly).
- Joined
- Dec 27, 2014
- Messages
- 658
We just put dry ice in little Styrofoam 6 pack coolers in the lab for keeping tissue samples and the like cold.
No need to use a solvent with it, stuff gets very cold just sitting on it.
If you want some real fun, get some liquid nitrogen. You can put it in a stainless thermos if you leave the lid a bit loose.
We always throw it on the floor when no one is looking after we are finished with it. Never gets old watching it boil
No need to use a solvent with it, stuff gets very cold just sitting on it.
If you want some real fun, get some liquid nitrogen. You can put it in a stainless thermos if you leave the lid a bit loose.
We always throw it on the floor when no one is looking after we are finished with it. Never gets old watching it boil
B
Bill Gruby
Forum Guest
Register Today
The freeze spray has the ability to fracture the bronze if it becomes too cold to fast. Don't ask me how I found that one out. It wasn't a pretty sight. Use the hot plate in conjunction with the freezer or Dry Ice.
"Billy G"
"Billy G"
Last edited by a moderator:
My Uncle used to raise cattle. He told me he used Dry Ice and rubbing alcohol for cold branding. Some grocery stores carry dry ice pieces in my area. Be careful with that mixture, causes skin damage in a few seconds without heavy gloves.

