That product has one of the worst, compliance-skirting, dirty corporate capitalist Safety Data Sheets I've ever seen, and I've reviewed thousands. It's water plus 5% we're not going to tell ya without a subpoena.
-
Welcome back Guest! Did you know you can mentor other members here at H-M? If not, please check out our Relaunch of Hobby Machinist Mentoring Program!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Lets try this rust remover again .
- Thread starter mmcmdl
- Start date
- Joined
- Apr 23, 2018
- Messages
- 6,532
I hate to say it, but he's wrong. The TEA-phosphate is the chelator, but the secret ingredient is the synthetic sulfur bearing terminal receptor. So he identified the garbage truck, but didn't identify the landfill. Without the receptor, the chelator would fill up and the solution would stop working rather quickly. I found some candidates for the sulfur bearing compound, but I think they're too simple, I'm guessing that the Evaporust receptor is a bit of a bulky, polymerized blob with a lot of sulfonated or sulfated sites for high capacity.

I’ll have to take your word for it!I hate to say it, but he's wrong. The TEA-phosphate is the chelator, but the secret ingredient is the synthetic sulfur bearing terminal receptor. So he identified the garbage truck, but didn't identify the landfill. Without the receptor, the chelator would fill up and the solution would stop working rather quickly. I found some candidates for the sulfur bearing compound, but I think they're too simple, I'm guessing that the Evaporust receptor is a bit of a bulky, polymerized blob with a lot of sulfonated or sulfated sites for high capacity.
View attachment 481569
- Joined
- Dec 9, 2021
- Messages
- 731
It was a surprise to me when I found out that Glyphosate (Roundup weed killer) was developed as a chelating agent. I don't know how well it worked for that application, but it seems that chemistry is kind of Black Magic, where everything is not as it seems, outcomes can vary depending on multiple factors, and unintended consequences abound.
- Joined
- Oct 17, 2023
- Messages
- 24
However Evaporust works it works very well.I hate to say it, but he's wrong. The TEA-phosphate is the chelator, but the secret ingredient is the synthetic sulfur bearing terminal receptor. So he identified the garbage truck, but didn't identify the landfill. Without the receptor, the chelator would fill up and the solution would stop working rather quickly. I found some candidates for the sulfur bearing compound, but I think they're too simple, I'm guessing that the Evaporust receptor is a bit of a bulky, polymerized blob with a lot of sulfonated or sulfated sites for high capacity.
View attachment 481569
Brilliant product IMO.
- Joined
- Sep 1, 2023
- Messages
- 468
I think the reason the reviews on Amazon (both .uk and .com) have, in addition to the majority 5 star reviews, a significant number of 1 star reviews, is that people just don't read and understand instructions properly.However Evaporust works it works very well.
Brilliant product IMO.
Removing muck oil and grease from the rusted items is really important and sticking Evaporust in a bucket in a cold garage, won't be particularly successful. The manufacturers say temperatures below 15 degrees Celsius reduce its effectiveness and to be honest I've found it works fastest and most effectively above 20 degrees Celsius.
- Joined
- May 7, 2023
- Messages
- 1,338
8 MONTHS?I have a few items I want to bring back to life . NO precision stuff , but doo dads and whatever . I used this on a chuck and soaked it for 8 months or so . Came out worse than when it went in . My fault for forgetting all about it . I'm comparing this to simple , cheap white vinegar this time . Will post results .
Wow, you got me beat. I left a part in the Boshield rust remover for a week and it corroded the hell out of it. Do not confuse this with their T-9 offering, T-9 is great.
The rust remover is so friggin harsh and poisonous I may just toss it.
A soak in Evaporust cleaned that hot mess up fine though.
- Joined
- Jan 2, 2014
- Messages
- 8,852
I'm guessing that the Evaporust receptor is a bit of a bulky, polymerized blob with a lot of sulfonated or sulfated sites for high capacity.
John,
I suspect I'll just have to nod along and whisper "right,....... magic", but thought I'd ask..........
Can you explain (hopefully in more basic language than above) how it pulls the iron from iron oxide, but leaves the iron in the base metal alone?
Is it just the way that the iron in the iron-oxide is available for chemical bonding?
Sort of a key and lock, or pattern of bond locations type thing and the iron-oxide presents differently than the other iron molecules mixed with carbon, manganese, nickels, chromium, molybdenum, etc.
Somehow I have a basic view of simple chemistry as "molecular hooks"; soaps hook to water and oils, etc.
I don't know if this idea was taught to me, or if it just something my brain threw together when trying to get thru the few, limited classes I took.
Got any book recommendations for low-level chemistry wanna-be types? Something between Bill Nye and my son's 20lb "Organic Chemistry" university text book.
Thanks for any guidance!
Brian
- Joined
- Apr 23, 2018
- Messages
- 6,532
John,
I suspect I'll just have to nod along and whisper "right,....... magic", but thought I'd ask..........
Can you explain (hopefully in more basic language than above) how it pulls the iron from iron oxide, but leaves the iron in the base metal alone?
Is it just the way that the iron in the iron-oxide is available for chemical bonding?
Sort of a key and lock, or pattern of bond locations type thing and the iron-oxide presents differently than the other iron molecules mixed with carbon, manganese, nickels, chromium, molybdenum, etc.
Somehow I have a basic view of simple chemistry as "molecular hooks"; soaps hook to water and oils, etc.
I don't know if this idea was taught to me, or if it just something my brain threw together when trying to get thru the few, limited classes I took.
Got any book recommendations for low-level chemistry wanna-be types? Something between Bill Nye and my son's 20lb "Organic Chemistry" university text book.
Thanks for any guidance!
Brian
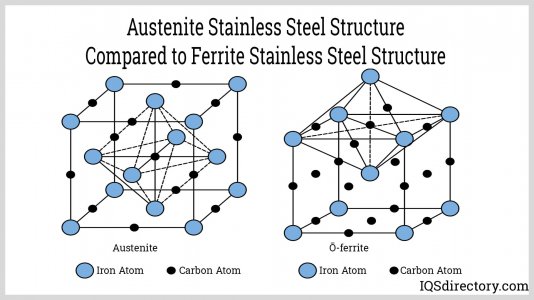
Yes, you are thinking along the correct lines. Chelation is based on charge affinity, the same way water gangs up on charged particles to form "spheres of solvation", except chelators are are flexible molecules with many charged sites that can wrap around oppositely charged atoms. Metals have numerous electron configurations that provide charged sites. Steel and alloys share their electrons in various packed lattices like these, which makes them resistant to weaker chemical action:
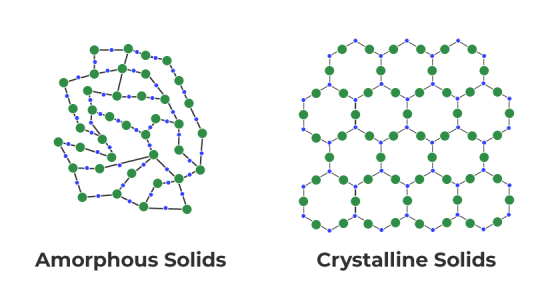
But rust is "amorphous" meaning it has no structure. it's just a blob of porous, mostly free oxides of iron that has charged sites that a chelator can grab onto, a lot like the key and lock example.
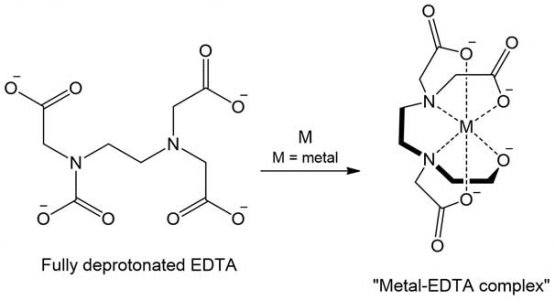
This is EDTA, a textbook chelator. Citric acid is similar, but not as strong (it takes two to fully chelate the 6+ example below):
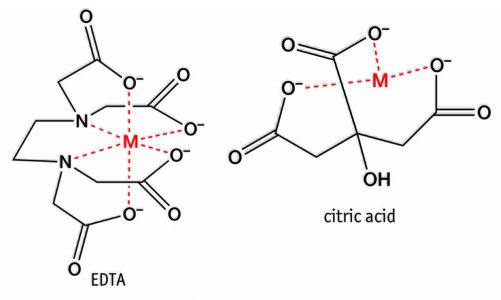
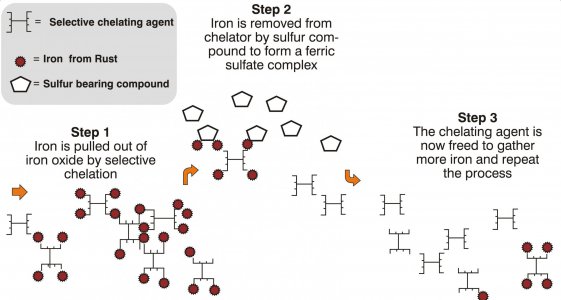
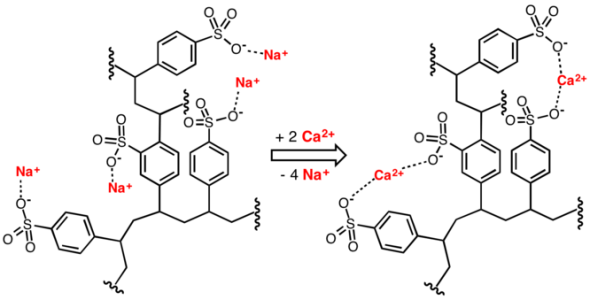
But once the chelator is bound, it's inerted and cannot perform further action, unless the metal passed on to something else, like the terminal receptor I was talking about. Alkyl sulfones make good terminal receptors, and if "tannic acid" is one of the ingredients, it would make sense that a lignin sulfonate exchange is how the chelating agent is "refreshed". Obviously, that is their trade secret. Just consider iron oxide as the particle, and instead of the ionic bond, it's a weaker interaction supported by many active sites working together. Iron oxide has enough charge to deprotonate water, so the iron oxide hydroxide-iron oxide receptor complex is driven by that.
- Joined
- Feb 26, 2021
- Messages
- 470
I hate to say it, but he's wrong. The TEA-phosphate is the chelator, but the secret ingredient is the synthetic sulfur bearing terminal receptor. So he identified the garbage truck, but didn't identify the landfill. Without the receptor, the chelator would fill up and the solution would stop working rather quickly. I found some candidates for the sulfur bearing compound, but I think they're too simple, I'm guessing that the Evaporust receptor is a bit of a bulky, polymerized blob with a lot of sulfonated or sulfated sites for high capacity.
You, sir, speak a language that I'm not familiar with! I trust you, though, so just tell me what to do?

