-
Welcome back Guest! Did you know you can mentor other members here at H-M? If not, please check out our Relaunch of Hobby Machinist Mentoring Program!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Casting aluminum on a backyard
- Thread starter r3292c
- Start date
- Joined
- Nov 1, 2018
- Messages
- 98
Here is a video about new casting I recently had.
In general, the part looks OK, but porosity on the machined surfaces is quite high. Any idea how to reduce porosity? Aluminum was pre-casted to "muffin" ingots, borax was used for degassing, casting temperature was 680C = 1256F. Regular green sand (silica sand plus bentonite clay) was used.
Any comments or suggestions are very welcome. I'm just learning things.
In general, the part looks OK, but porosity on the machined surfaces is quite high. Any idea how to reduce porosity? Aluminum was pre-casted to "muffin" ingots, borax was used for degassing, casting temperature was 680C = 1256F. Regular green sand (silica sand plus bentonite clay) was used.
Any comments or suggestions are very welcome. I'm just learning things.
- Joined
- Aug 22, 2012
- Messages
- 4,260
My last cast was one of my most solid, ie no porosity visible.
I used my muffin tin ingots but barely let them melt before pouring.
check out post 210 for pics

 www.hobby-machinist.com
I'm also wondering if too much gas, ie too rich a flame adds to porosity, still testing this though.
www.hobby-machinist.com
I'm also wondering if too much gas, ie too rich a flame adds to porosity, still testing this though.
I used my muffin tin ingots but barely let them melt before pouring.
check out post 210 for pics

The Giant Binocular
Sav, have you looked at the various wetsuit repair kits available, might find something useful at a SCUBA shop. Must be a few in your area of the world. John. Yes, they are for sticking neoprene to neoprene. Works well for that but doesnt stick to the glass or aluminium. I will experiment today...
 www.hobby-machinist.com
www.hobby-machinist.com
- Joined
- Mar 25, 2013
- Messages
- 5,108
Gas porosity in Al is related to solution of hydrogen gas. It comes out of solution as the metal solidifies forming bubbles. The melt is always exposed to hydrogen in a combustion furnace. Even water can break down and be absorbed as hydrogen.
Borax is a flux, not to degas. In fact it may have water in it and contribute to porosity. I would not use any flux unless you have dirty metal or some known issue that requires it. Melting your aluminum into ingots first is also a bad idea. Every time you melt the metal you pick up more porosity. The best way to prevent porosity is to use clean metal, melt it quickly and pour as soon as possible. The melt will be absorbing hydrogen the whole time it is liquid. Degassing is possible but very cumbersome in a home foundry.
I am convinced that running a furnace lean (oxidizing environment) is helpful but others have argued I was wrong on this. Take that with a grain of salt.
I also think electric furnaces are better is this regard since they do not produce hydrogen. You can even inert them with Ag if you wish.
Not trying to sound critical. I hope that helps.
Robert
Borax is a flux, not to degas. In fact it may have water in it and contribute to porosity. I would not use any flux unless you have dirty metal or some known issue that requires it. Melting your aluminum into ingots first is also a bad idea. Every time you melt the metal you pick up more porosity. The best way to prevent porosity is to use clean metal, melt it quickly and pour as soon as possible. The melt will be absorbing hydrogen the whole time it is liquid. Degassing is possible but very cumbersome in a home foundry.
I am convinced that running a furnace lean (oxidizing environment) is helpful but others have argued I was wrong on this. Take that with a grain of salt.
I also think electric furnaces are better is this regard since they do not produce hydrogen. You can even inert them with Ag if you wish.
Not trying to sound critical. I hope that helps.
Robert
Last edited:
- Joined
- Nov 1, 2018
- Messages
- 98
Hello Robert, thanks for you reply
Yes, I understand the porosity (gas porosity) might come from Hydrogen, out of the water: Al + H2O = Al2O3 + H2
H2O may come from flux, or from baking soda (some people use). As well as from propane burning, even from green sand (we add water into it). I keep my borax in a zip lock bag to limit amount of water in it. Added to crucible with melted aluminum, borax creates lots of bubbles. I plunge borax to the bottom of crucible, thinking it acts like degassing agent, bubbling through aluminum. Maybe my understanding isn't correct.
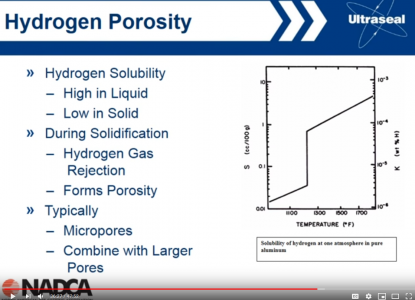
About re-melting aluminum. I'm not an expert, but I heard re-melting helps reducing porosity. When the aluminum solidifies, it pushes hydrogen out. So, once aluminum solidifies, the amount of hydrogen in it is reduced by an order - see screenshot. This means, even porous ingot has much less hydrogen dissolved than shiny melted metal.
I agree with you that electric furnaces are better, clean metal should be used, melt it quickly and pour as soon as possible.
Yes, I understand the porosity (gas porosity) might come from Hydrogen, out of the water: Al + H2O = Al2O3 + H2
H2O may come from flux, or from baking soda (some people use). As well as from propane burning, even from green sand (we add water into it). I keep my borax in a zip lock bag to limit amount of water in it. Added to crucible with melted aluminum, borax creates lots of bubbles. I plunge borax to the bottom of crucible, thinking it acts like degassing agent, bubbling through aluminum. Maybe my understanding isn't correct.
About re-melting aluminum. I'm not an expert, but I heard re-melting helps reducing porosity. When the aluminum solidifies, it pushes hydrogen out. So, once aluminum solidifies, the amount of hydrogen in it is reduced by an order - see screenshot. This means, even porous ingot has much less hydrogen dissolved than shiny melted metal.
I agree with you that electric furnaces are better, clean metal should be used, melt it quickly and pour as soon as possible.

- Joined
- Mar 25, 2013
- Messages
- 5,108
I don't think Borax is appropriate to degas. Some people use chlorine pool tablets which scavenge the hydrogen. Rotary degassing with inert gas is used in industry.
I disagree about remelting. I think it makes porosity worse. You should consider becoming a member at Alloy Avenue. If you search over there for porosity you will find some detailed discussions. Those discussions with experienced casters are the basis for my advice. Another good site is http://forums.thehomefoundry.org/index.php
Robert
I disagree about remelting. I think it makes porosity worse. You should consider becoming a member at Alloy Avenue. If you search over there for porosity you will find some detailed discussions. Those discussions with experienced casters are the basis for my advice. Another good site is http://forums.thehomefoundry.org/index.php
Robert
