-
Welcome back Guest! Did you know you can mentor other members here at H-M? If not, please check out our Relaunch of Hobby Machinist Mentoring Program!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
DIY Blue Spotting Blues!
- Thread starter graham-xrf
- Start date
- Joined
- Apr 23, 2018
- Messages
- 6,602
The oils that you are talking about are called triclycerides. The reaction that @homebrewed described is called saponification (from the greek word Sapphos), where the glycerol is cleaved from the ester linkages of the fats in the presence of strong base. That's followed by transesterification, where H+ from water caps the fats, creating a fatty acid that has an amphoteric property (one end is liposoluble, the other is water soluble). The same reaction can also be performed with strong acid. This is indeed how you make soap and glycerin in the cookpot. What it all means depends on the result you are trying to attain. Different oils, different catalysts, different temperatures, different endpoints. Big rabbithole.
- Joined
- May 27, 2016
- Messages
- 3,480
I kind of guessed from looking at formula C3H8O3 that there was a whole lot of H fuel in there. It's the bones of good ole' "nitro".
I am happy to duck the business of dripping fuming H2SO4 into caster oil. Getting the acid stuff here is a bit fraught also, unless I were to boil up the 2L of battery acid! The UK soap shop to the rescue, and it seems affordable.
Yes - I have wondered what would happen if I just mixed some blue into some soap, but I already guess that that dog likely won't hunt! So far we have ..
1. Sulfonated caster oil (Red Turkey)
2. Ptalocyanine blue pigment
3. Titanium Dioxide Pigment (Chinese White)
The first three we have got some supplies. Then we get to the one's I don't know.
- - - - - - - - - - - - - -
4. Sorbitan, Monooleate Polyxyetylene Deriv
It brings up lots of hits on the net. It's a food additive E494. A fatty acid ester.
Also used for "iron ore flotation" ???
Allegedly "has a special degree of esterification capable of obviously improving the dispersibility and water solubility of sodium soap" I have no idea what to use here - or to leave it out.
"Suitable for vegetarian" label indicates only vegetable fat used as a source.
E494 - Sorbitan monooleate: Produced from sorbitol and lauric acid, a normal fatty acid from vegetable or animal origin. Emulsifier, stabiliser stopping yeast products foaming. Banned in Australia and some other countries. Used in pharmaceuticals.
No idea where one could obtain some.
- - - - - - - - - - -
5. Nonyl Phenol Emulsifier
Non-ionic surfactant. I dunno. From what it does, it sounds like washing-up liquid, or maybe clothes wash.
I would be tempted to try Fairy Liquid Wash-up stuff in it's place.
Clearly @pontiac428, @hman , and @homebrewed managed to stay awake during Chem3.x
I am happy to duck the business of dripping fuming H2SO4 into caster oil. Getting the acid stuff here is a bit fraught also, unless I were to boil up the 2L of battery acid! The UK soap shop to the rescue, and it seems affordable.
Yes - I have wondered what would happen if I just mixed some blue into some soap, but I already guess that that dog likely won't hunt! So far we have ..
1. Sulfonated caster oil (Red Turkey)
2. Ptalocyanine blue pigment
3. Titanium Dioxide Pigment (Chinese White)
The first three we have got some supplies. Then we get to the one's I don't know.
- - - - - - - - - - - - - -
4. Sorbitan, Monooleate Polyxyetylene Deriv
It brings up lots of hits on the net. It's a food additive E494. A fatty acid ester.
Also used for "iron ore flotation" ???
Allegedly "has a special degree of esterification capable of obviously improving the dispersibility and water solubility of sodium soap" I have no idea what to use here - or to leave it out.
"Suitable for vegetarian" label indicates only vegetable fat used as a source.
E494 - Sorbitan monooleate: Produced from sorbitol and lauric acid, a normal fatty acid from vegetable or animal origin. Emulsifier, stabiliser stopping yeast products foaming. Banned in Australia and some other countries. Used in pharmaceuticals.
No idea where one could obtain some.
- - - - - - - - - - -
5. Nonyl Phenol Emulsifier
Non-ionic surfactant. I dunno. From what it does, it sounds like washing-up liquid, or maybe clothes wash.
I would be tempted to try Fairy Liquid Wash-up stuff in it's place.
Clearly @pontiac428, @hman , and @homebrewed managed to stay awake during Chem3.x
Attachments
Last edited:
- Joined
- Apr 23, 2018
- Messages
- 6,602
One thought on the Polysorbate 80 is its purpose as an emulsifier- you could try a small amount of propylene glycol and muddle in some xanthan gum, then mix in your pigment to obtain the texture you want. This will work and will let you order your supplies from Amazon instead of dealing with any hazmat or proprietaries.
- Joined
- Jul 28, 2017
- Messages
- 2,393
What he said (much more precisely than I).The oils that you are talking about are called triclycerides. The reaction that @homebrewed described is called saponification (from the greek word Sapphos), where the glycerol is cleaved from the ester linkages of the fats in the presence of strong base. That's followed by transesterification, where H+ from water caps the fats, creating a fatty acid that has an amphoteric property (one end is liposoluble, the other is water soluble). The same reaction can also be performed with strong acid. This is indeed how you make soap and glycerin in the cookpot. What it all means depends on the result you are trying to attain. Different oils, different catalysts, different temperatures, different endpoints. Big rabbithole.
- Joined
- Jul 28, 2017
- Messages
- 2,393
One aspect of the TiO2 component to think about before adding it to your DIY spotting compound. There are two commonly-available forms that have different crystal structures and very different hardness. They are the rutile and anatase forms. Rutile is very hard, anatase is relatively soft. To avoid potential issues with wear on your granite surface plate, I'd suggest using the anatase form. Soapgoods used to sell the anatase form but when I checked today, I didn't find any indication of what form they sell (not even in the SDS). Or you could just try leaving it out and seeing how your spotting compound works without it.
Alternatives to TiO2 could be zinc oxide or barium sulfate. Both are relatively soft, and, like TiO2, insoluble in water. Soapgoods sells zinc oxide powder, and your source for sulfonated castor oil probably does, too.
Variations in crystal structure are often associated with variations in hardness. In addition to diamond vs graphite, boron nitride has two forms. One is nearly as hard as diamond and the other can be used as a dry lubricant! Best not to mix the two up, eh?
Alternatives to TiO2 could be zinc oxide or barium sulfate. Both are relatively soft, and, like TiO2, insoluble in water. Soapgoods sells zinc oxide powder, and your source for sulfonated castor oil probably does, too.
Variations in crystal structure are often associated with variations in hardness. In addition to diamond vs graphite, boron nitride has two forms. One is nearly as hard as diamond and the other can be used as a dry lubricant! Best not to mix the two up, eh?
- Joined
- May 27, 2016
- Messages
- 3,480
I didn't know about hardness. There are two more, being akaoglite and brookite.
The descriptions of it eBay only sometimes put "(rutile)" in brackets. No other kind is mentioned.
I think if TiO2 in any form is heated up 800°+, it turns into rutile.
The one I got was --> THIS
Somewhat cheaper, but actually mentioning rutile was THIS#2
The 500g packet came to £7.69, but I wanted in the HDPE bottle with a cap.
I think TiO2 rutile may be like corundum Al2O3 I guess when it arrives, I try it (as abrasive).
I know it's used along with caster sugar in cake icings, and in paint "brilliant white".
I would have thought that hard abrasive is the last thing we want on our precious granite surface plate. When we do spotting, and that little rub to see the swing point, we would be in effect abrasive lapping. No wonder toothpaste is such a good metal polish!
The descriptions of it eBay only sometimes put "(rutile)" in brackets. No other kind is mentioned.
I think if TiO2 in any form is heated up 800°+, it turns into rutile.
The one I got was --> THIS
Somewhat cheaper, but actually mentioning rutile was THIS#2
The 500g packet came to £7.69, but I wanted in the HDPE bottle with a cap.
I think TiO2 rutile may be like corundum Al2O3 I guess when it arrives, I try it (as abrasive).
I know it's used along with caster sugar in cake icings, and in paint "brilliant white".
I would have thought that hard abrasive is the last thing we want on our precious granite surface plate. When we do spotting, and that little rub to see the swing point, we would be in effect abrasive lapping. No wonder toothpaste is such a good metal polish!
- Joined
- Feb 6, 2020
- Messages
- 257
Black soot mixed with way oil - greasy but it worked better then Permatex
black soot mixed with linseed oil and a binder. GM formula for older wood truck beds. one of mine is done that way and is still like new 15 years later
- Joined
- Jul 28, 2017
- Messages
- 2,393
I did say "commonly-available"I didn't know about hardness. There are two more, being akaoglite and brookite.
The descriptions of it eBay only sometimes put "(rutile)" in brackets. No other kind is mentioned.
I think if TiO2 in any form is heated up 800°+, it turns into rutile.
The one I got was --> THIS
Somewhat cheaper, but actually mentioning rutile was THIS#2
The 500g packet came to £7.69, but I wanted in the HDPE bottle with a cap.
I think TiO2 rutile may be like corundum Al2O3 I guess when it arrives, I try it (as abrasive).
I know it's used along with caster sugar in cake icings, and in paint "brilliant white".
I would have thought that hard abrasive is the last thing we want on our precious granite surface plate. When we do spotting, and that little rub to see the swing point, we would be in effect abrasive lapping. No wonder toothpaste is such a good metal polish!
But on the other hand, diamonds fall into that category but aren't commonly affordable!
Going back to Richard's comment and #38 in this thread, If you have a used laser printer cartridge lying around you probably have a small quantity of very fine black pigment to play with. More "fun" stuff to experiment with.
- Joined
- May 27, 2016
- Messages
- 3,480
Oh yes. Using carbon black did not escape my attention, and used laser printer cartridges can easily be found. Even simply purchasing some carbon black is OK. I did see on YT the technique of two colours, one black, and the other red. I forget which way around, or maybe it doesn't matter, but you get a high contrast set of spots. I think a very thin, near transparent red is put on the scrape metal, and a black on the surface plate, to give black spots on a red background.black soot mixed with linseed oil and a binder. GM formula for older wood truck beds. one of mine is done that way and is still like new 15 years later
This is also done with yellow, and blue. I think that's why Canode, when it could be had, came in those colours.
eBay mistaike
Here is one of those eBay mess-ups. I sometimes wonder what would happen if someone tried to pay for obvious mistake. These two items are from the same seller, to whom we award zero points for consistency. It seems 100g of soot costs £6.50 ($8.66).
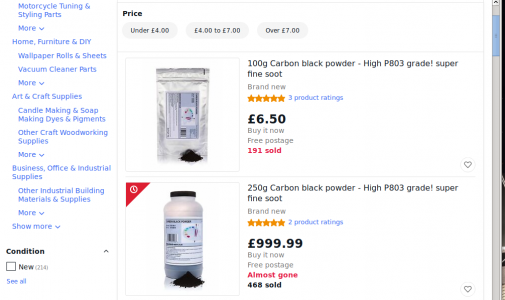
So - still punting for "Hobby Machinist Tightwad of the Year", I opt to go dumpster-diving for ex-laser printer soot!
Last edited:

